- europages
- >
- CAD CAM Computer Assisted Design Computer Assisted Manufacturing - software
- >
- ENTEKNOGRATE CORP.
- >
- FEA & CFD based Simulation & Design: Medical & Biomedical
FEA & CFD based Simulation & Design: Medical & Biomedical
with FDA's guidance for Reporting in in Medical Device Submissions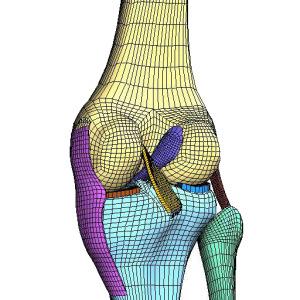
Description
FEA and CFD based Simulation design and analysis is playing an increasingly significant role in the development of medical devices, saving development costs by optimizing device performance and reliability, reducing benchtop tests and clinical trials, and helping to speed the regulatory approval process. Developing increasingly complex medical devices requires increasingly capable tools for simulation and testing. Our deep portfolio of simulation and testing solutions allows medical device developers to generate digital evidence of device performance across a range of engineering disciplines, throughout the product life cycle. Medical applications are typically subjected to a wide range of complex environmental and biological loading conditions.The variability of these conditions makes the physical testing of all possible scenarios both difficult and time-consuming. By using FEA and CFD multidisciplinary and multiphysics simulation technology, our engineers can study a greater number o
- CAD/CAM Computer Assisted Design/Computer Assisted Manufacturing - software
- FEA
- CFD
- Ansys
Similar products
EC PLAZA
South Korea
Description - Small and medium sized shipyard customized ERP system - Low-cost/high-efficiency systems can be utilized to secure competitiveness in orders through cost reduction. - Maximize production efficiency through cloud-based drawing management Automatically extract BOM based on 2D drawings - Minimize waste of time and resources by linking materials management and ordering systems Features · Material specification storage, data call function, design drawing (dxf) registration view function. · Generation of POR (Purchase Request) through BOM linkage, conversion to data slip form for each department, and order management through integration (Inventory inquiry) · Implement a history function that enables follow-up by data change process. · Report and enter sales management information such as order, shipment, estimate, and collection information in real time. · Processing by project and task and uploading work data by department.
Request for a quote
GRAVOTECH
France
For over 30 years, TYPE EDIT has been the complete CAD/CAM software solution. Taking your ideas and drawings, through design and machining to production. - WITH 400+ POWERFUL FEATURES within 3 interactive modules, TYPE EDIT is packed with vector creation and manipulation tools, combined with 3D bas-relief, connected to a powerful CAM engine creating the smartest toolpath strategies. - COMPATIBLE WITH Unicode, 64 bit, and available in 20+ languages. TYPE EDIT is used across many sectors and applications. - APPLICABLE Engrave, Mark, Cut, Carve in 2D, 2.5D, and 3D within hard metals or soft materials, on any CNC milling machine.
Request for a quote
RAYUEN PACKAGING CO.,LIMITED
China
cylinder wooden perfume cap with arcshaped top, real wooden cover assembled with plastic PP inner cap, can fit with FEA 15 perfume bottle with crimp sprayer as luxury perfume packaging. Decoration available custom shape or style, color painting in the glossy and matte finish, laser engraved logo etc.
Request for a quoteRequest for quotes
Create one request and get multiple quotes form verified suppliers.
- Only relevant suppliers
- Data privacy compliant
- 100% free
