- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- responsible person
Results for
Responsible person - Import export

TAOBÉ CONSULTING
Belgium
The “Responsible Person” is a vital figure in the beauty and cosmetics sector, acting as the primary contact for ensuring cosmetic goods are in line with the rigorous safety and regulatory standards established by the EU frameworks. Only cosmetic products for which a natural or legal person has been designated as a ‘responsible person’ shall be placed on the market. The obligations of the Reponsible Person are: •to ensure that the Safety Assessment of the cosmetic has been carried out by a Safety Assessor, •to maintain specific information on the product it places on the market and make sure it remains up to date. •to handle the inspections carried out by the authorities; •to perform regulatory watch to make sure the PIF remains compliant throughout its lifetime on the market Trust professionals to handle this crucial role with care and give you peace of mind.
Request for a quote
ROTAS SWORN AND ORDINARY TRANSLATION OFFICE
Poland
We can offer our clients the rental of analog mixers that will prove themselves during the organization of conferences or small concerts. The Soundcraft EPM6 analog mixers offer 6 mic inputs, 2 stereo TRS inputs and 2 AUX sends. The devices are characterized by high sound quality and ease of use, so there is no need to involve a person responsible for technical service. The set, in addition to the blender in a cover, also includes a power cord.
Request for a quote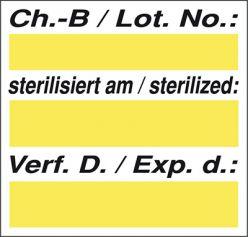
METO INTERNATIONAL GMBH
Germany
Meto sterilisation labels to indicate that medical instruments have been sterilised. Identification, monitoring and documentation with a label. Meto sterilisation labels facilitate reliable process control for medical sterilisation using steam, ethylene oxide and gamma rays thanks to a permanent change in the colour of the indicator ink. It is also possible to print out additional data such as the steriliser, person responsible, date, batch number and use-by date.
Request for a quote
POLE COSMÉTIQUE
France
The import declaration of cosmetic products from a country outside of the European Union is subject to strict regulation which involves the following three compulsory steps: Registration of the importing company (in France, the company should be registered at ANSM, formerly AFSSAPS, by appointing qualified responsible persons) Preparation of the product information file for each product you import (formula) Notification of your products to the European Commission; henceforth possible with the European Cosmetic Products Notification Portal (CPNP) One of the biggest challenges with importing a product into E.U. is the lack of required qualifications. Qualifications are required to be responsible towards the National Agency for Medicinal and Health Product Safety (ANSM), but also for the safety assessment included in the Product Information File (PIF). Pôle Cosmétique makes your import project easier by taking charge for definition of the PIF and notification of the product to the...
Request for a quote
TAOBÉ CONSULTING
Belgium
Before a cosmetic can be sold in Europe (31 countries) or in the UK, it needs to be notified on the Cosmetic Product Notification Portal if the product is intended to be sold in Europe and on the Submit Cosmetic Product Notifications if the target market is Great Britain. After the notification, the product will receive a unique number that will allow customs and authorities to identify it clearly. It is usually the Responsible Person who notifies the product on its own CPNP or SCPN Account.
Request for a quote
TAOBÉ CONSULTING
Belgium
All cosmetic products must display the following mandatory information on both the container (inner label) and packaging (outer label): - Name and address of the Responsible Person - Nominal content - Date of minimum duration or PAO - Country of origin (if the product is imported into the community) - Product function (unless it’s clear from its presentation) However, there are exceptions for the following three items, which we will discuss in detail later in this article. - Warnings - Batch number - INCI list Please keep in mind that the items mentioned above are specified in EU Regulation 1223/2009, which outlines the requirements for cosmetics. However, it’s important to understand that various laws govern product labelling, such as the Packaging & Waste regulations, which can vary from country to country. We encourage you to explore our article on Labelling and Packaging Regulations for more information, including details about recycling symbols.
Request for a quote
TAOBÉ CONSULTING
Belgium
Candles and non-combustible air fresheners are subject to specific categorization under EU regulations, aligning with efforts to ensure the safety and quality of consumer goods. To ensure the safety of products on the market, it is important to meet all compliance standards. Taobé’s experts will guide you through the CLP Regulation, the standards related to the labelling and safety testing of your candles (EN 15494:2019, EN 15493:2019, EN 15426:2018), and the Unique Formula Identifier, UFI, that will need to be included on the product’s label (crucial in notifying your product through the Poison Center Notification portal for the markets in which your candles will be available for sale). Taobé extends its Responsible Person services agreement to cover general use products, ensuring compliance with the new General Product Safety Regulation.
Request for a quote
GALEX D.O.O.
Slovenia
We prepare the entire PIF (product information file) documentation for you. A PIF is a collection of documents describing a cosmetic product. It includes a risk assessment for all substances, signed by a qualified professional. After carrying out all the required studies, each cosmetic product must be reported to the European Commission on the CPNP (Cosmetic Products Notification Portal). It is also obligatory to report the person responsible for placing the product on the market. In the case of dealing with food supplements, we prepare all the documentation with the content of individual active ingredients contained in the product. We also review all documentation of input subtances.
Request for a quote
A&T FORMULATION LTD.
United Kingdom
Compiling of a Product Information File (PIF) CPNP registration Safety Assessment Cosmetic Product Safety Report (CPSR) Designation of a responsible person within the EU Cosmetic labeling review Launch of products on the UK market Launch of products on the EU market Launch of products on the US market Expert support for entry into other markets
Request for a quote
HEMOGUM
Serbia
For the production and application of industrial goods in car industry we follow the SRPS ISO 9001-2008 ISO norm as well as TRV norm.This understands the specific application during technical production and personal responsability in the use of specific materials for the production of goods used in car industry. We produce air tubes, fuel tubes and oil tubes, shock absorbers, bearers, membranes, floor mats, protection caps resistant to grease and oils... Hemogum can meet car industry customers in production of articles for all renowned car manufacturers. We have the necessary equipment that helps us guarantee high quality, uniformity, excellent physical characteristics and high dimensional precision of our articles. For the production of car industry goods we apply the NR, CR, SBR, NBR, MWQ, FWK
Request for a quote
COLLETT & SONS LTD
United Kingdom
> Click on the "WEBSITE" link for more > With our modern range of equipment and Team of experienced operatives your lifting requirements will always be safe in our hands. Our dedicated team of Heavy Lift Engineers possess the expert skills and knowledge required to manoeuvre, position, extract and relocate cargoes utilising our Crane Hire, Contract Lift and Jacking & Skidding services. Undertaking all lifting responsibilities our in-house Appointed Persons, Slinger, Banksmen and Crane Lift Supervisors, operating under Contract Lift Conditions, can provide Crane Hire services directly tailored to our client's individual requirements. Coupled with this, and ahead of the commencement of any lifting operations, our Team provide all the necessary surveys, lift plans, method statements and risk assessments to ensure maximum safety at all times. Where Crane Hire is not a suitable option we operate a hydraulic lifting gantry or for working in more confined spaces.
Request for a quote
WATER HYGIENE CENTRE LTD
United Kingdom
This course deals with the legal & management aspects of water safety and is accredited with CPD & recognised by ILM, part of the City & Guilds Group. The term ‘water safety’ is a broad capture for risks associated from Legionnaires’ disease / Scalding / Pseudomonas aeruginosa [Pa]. The approach to managing these risks will depend on the type of organisation and the types of water risk systems and susceptibility of those who are exposed. Topics covered on the course will include: - Background to Legionella / Scalding / Pseudomonas aeruginosa - Susceptibilities - Proven & frequent sources of Legionnaires’ disease & Pseudomonas aeruginosa - Ecology of waterborne pathogens - Control strategies for managing water safety risk - Understanding law, regulations & guidance - Best Practice - Prosecutions examples and lessons learnt - Development of a Water Safety Plan - Challenges facing organisations
Request for a quote
WATER HYGIENE CENTRE LTD
United Kingdom
The audit process draws on the management requirements of the HSE's ACoP (L8) and HTM 04-01 & HTM 03-01 (where appropriate). If you are prosecuted for breach of health & safety law, and it is proved that you did not follow the relevant provisions of the Code, you will need to show that you have complied with the law in some other way or a Court will find you at fault.” A water safety audit is crucial to the development or refinement of any good risk management system & the control of Legionella. Our water hygiene audit will examine and verify the management systems you currently have in place & cover issues including: - Appointment of Responsible Persons' & associated staff - Roles, responsibilities & lines of communication - Risk assessment & re-assessment of compliance - Review of policy & procedures document(s) including records - Implementation of remedial works - Evidence of monitoring & performance of systems - Staff training
Request for a quote
EMERSON TRAINING SERVICES
United Kingdom
It is a legal requirement under The Lifting Operations and Lifting Equipment Regulations (LOLER) 1998 that every lifting operation is carried out in a safe manner. This includes use of a responsible person to guide the crane using suitable means of communication, and to securely attach the load to the crane. We have now developed a 'Foundation Plus' course which is five days in duration. This will give candidates more practical training time to give greater preparation for the CPCS Practical Test, and to enhance post-course knowledge and skills. With effect from January 19th 2015 this category has been amended to add endorsements against a range of lifting equipment types to cover the increased use of slingers for a wider variety of lifting operations. The endorsements are: A – All Types – all duties B – All Types – static duties C – Knuckle boom static only D – Excavator only E – Lift truck only
Request for a quote
MAXCERT LTD
Germany
The new European Commission’s guidance on persons responsible for regulatory compliance (PRRC) explains that manufacturers, Authorized Representatives and micro and small manufacturers must designate at least one staff member responsible for ensuring compliance to the MDR and/or IVDR as appropriate according to Article 15 of the Regulation. PRRC qualifications are specific for each of these three operators. This does not mean that you have to hire PRRC as employee rather you can avail our PRRC service to overcome the regualtory needs of your company and medical devices – with a consultant managing the system on a few days per month basis. We would be more than happy to discuss this option with you, helping you to find a consultant with the necessary qualifications to perform this service effectively. If you would like to know more about this service, then please contact us.
Request for a quote
BELAB SERVICES
Spain
Cosmetic product registration in Europe acting as a Responsible Person. PIF and Safety Assessment management.
Request for a quote
BELAB SERVICES
Spain
Registration of cosmetic products in the UK acting as a Responsible Person. PIF and Safety Assessment management.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Responsible person - Import exportNumber of results
17 ProductsCountries
Company type